Abstract
A readily available γ-glutamyl transfer reagent, namely, N-phthaloyl-L-glutamic anhydride (NPGLA) offers a simple solution for the racemization free synthesis of γ-glutamyl amino acids with usefully enhanced properties
γ-glutamyl dipeptides (γ-Glu AA) are defined in this context as the compounds derived by the acylation of an amino acid (AA) through the γ-carboxyl carbon of L-glutamic acid. The resulting amide linkage has sometimes been referred as a pseudo-peptide bond. The general structure of a typical γ-glutamyl dipeptide is given in Figure 1. Interest in γ-Glutamyl peptides and analogs has persisted for long (1). The aim of this short review is to highlight the useful improvement of properties of the amino acid which is γ-glutamylated. Also a brief review of the chemistry leading to γ-glutamyl dipeptide in a practical and efficacious way requiring the least number of protection/deprotection steps is presented.
LFigure 1. γ-Glutamyl amino acid(γ-Glu AA or γ-Glutamyl dipeptide).
Enzymes belonging to the class called γ-glutamyl transpeptidases lead either to the formation of this pseudopeptide bond or the hydrolysis of the γ-glutamyl dipeptide into its constituent amino acids depending on conditions.
Impact of γ-Glutamyl substructure on properties of amino acids
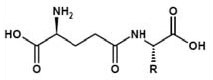
Suzuki et al found (2) that the bitter taste of valine was highly attenuated on transformation to its γ-glutamyl derivative. Similar glutamylation has been carried out on isoleucine and tyrosine.
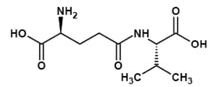
Figure 2. γ-Glutamylvaline (γ- Glu Val)
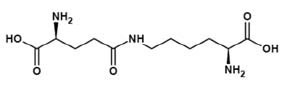
Figure 3. NÂ-(γ-Glutamyl)lysine
Ne-(γ-Glutamyl)lysine (Figure 3) is important amino acid component forming part of food products. Nutritional improvement of bread has been noticed with Ne-(γ- Glutamyl)lysine (3) wherein. Ne-(γ-Glutamyl)lysine serving as a nutritional source of lysine. In another study it was found that scanning electron microscopy indicated that the physical properties of dry noodles were improved through the formation of cross-links, Ne-(γ-Glutamyl)lysine, brought about by MTGase (4). Molecules (Figure 2 and Figure 4) possessing the properties of mouthfulness and thickness and increasing continuity of food taste perception have been termed by the Japanese as "kokumi" flavor compounds and such properties are ostensibly conferred by the γ-glutamyl residue on the amino acids (5). Glutathione, itself a γ-glutamyl peptide, γ-Glu-Cys-Gly, is also a "kokumi" compound. Another mouthfulness enhancing "kokumi" compound with a γ-glutamyl substructure is γ-Glutamyl-trans-S-propenyl-L-cysteine sulfoxide (Figure 5) occurring in garlic and onion.
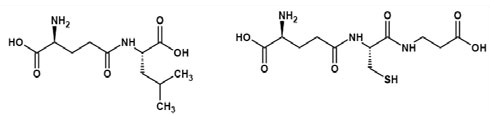
Figure 4. γ-Glutamylleucine and Homoglutathione.
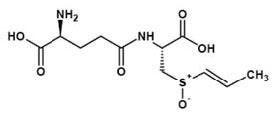
Figure 5. γ-Glutamyl-trans-S-propenyl-Lcysteine sulfoxide.
Occurrence of these γ-glutamyl "kokumi" compounds attest to the use of beans, garlic, onion being served often with meat dishes for enhancing the sensory perception as well as a longer lasting food sensation.
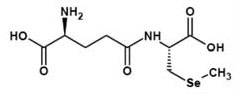
Figure 6. γ-Glutamyl-Se-methyl-Lselenocysteine.
Selenium amino acids rank among the top of the list on selenium supplements. Selenium has been recognized as an essential micronutrient the lack of which leads to various disease states (6), ascribed to the deficiency of selenium containing amino acids in the body. Certain selenium compounds are offensive in smell and this property may become a deterrent factor for oral and topical use. For example, while Se-Methyl-L-selenocysteine has an odor associated with it, the γ-glutamyl derivative is practically odorless (Figure 6). Still the biological characteristics of Se-methyl-Lselenocysteine are not considerably changed by this structural variation. It has been well recognized that γ-glutamyl-Se-methyl-L selenocysteine is a carrier molecule as effective as its parent, Se-methyl-L-selenocysteine, in its anticancerous properties (7). This improvement of olfactory property has another important application in cosmetics. While the not-so-acceptable smell of some of the selenium compounds may have contributed to a slightly muted acceptance of selenium compounds in cosmetic industry, olfactory improvement by γ-glutamylation should lead to a favorable acceptance of these compounds by the industry.
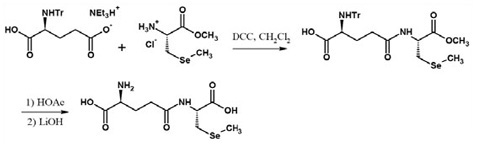
Figure 7. Typical Sequence for γ-glutamylation of amino acids
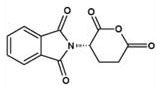
Figure 8. N-phthaloyl-Lglutamic anhydride (NPGLA). γ-GLUTAMYLATION AND PHYSICAL PROPERTIES
Since γ-glutamylation introduces more polar functional groups with hydrophilic characteristics, it can be anticipated that the solubility of γ-glutamyl amino acid in water may be enhanced compared to the corresponding underivatized amino acid. Such has been the case in the duo, Se-methyl- L-selenocysteine and its glutamylated product, γ-Glutamyl- Se-methyl-L-selenocysteine. The solubility of the L-Semethylselenocysteine is ca. 10 percent with dissolution occurring in two hours in water. The solubility of γ-L-glutamyl- L-Se-methylselenocysteine is ca. 25 percent with dissolution occurring in thirty minutes in water. This solubility modifying characteristic has important ramifications on the flexibility of formulation of these materials.
Miscellaneous Examples
Amino acids such as γ-glutamyl-taurine (Glutaurine) isolated from bovine parathyroids has been reported to influence the metabolism of Vitamin A and to possess radioprotective properties in addition to other useful therapeutic properties. Again such as an array of beneficial properties would appear to result from the modification of the taurine structure with γ-glutamyl group appendage (8). γ-D-Glutamyl- L-tryptophan (SCV-07) is a prospective medicine for the treatment of tuberculosis, that reached upto phase II clinical trial. Here the optical antipode (D) is used (9). Another stellar example is Theanine. Even though it is not a γ-glutamyl aminoacid, rather a γ-glutamyl ethyl amine, the γ-glutamyl structural fraction is the most important feature ofTheanine. γ-Glutamyl derivatives have been well recognized for their immunomodulating properties. Several amino acid and amine analogs have been described in the literature.
CHEMISTRY OF γ-GLUTAMYLATION
γ-Glutamyl group can be incorporated onto a typical amino acids in a several ways. Basically this usually requires the protection of two of the three carboxylic groups in the reactant molecules as esters (leaving of course the third γ-COOH of the glutamic acid free). Also the amino group of the L-glutamic acid residue should be suitably protected. Figure 7 shows a sequence wherein only two protecting groups are used. The trityl group on the glutamic acid functions plays a dual role wherein it protects the amino group as well as rendering the neighboring -COOH as sterically unreactive. The carboxyl of the other component, namely, Se-methyl-L-selenocysteine is protected as a methyl ester. After the formation of pseudopeptide bond, the two protecting groups need to be removed sequentially without affecting the amide linkage (10).
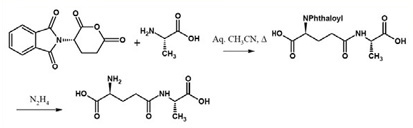
Figure 9. Reaction Sequence for γ-Glutamylation on Unprotected Amino Acids using. NPGLA
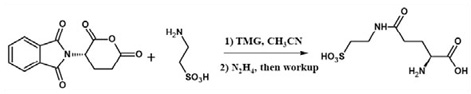
Figure 10. Single Pot γ-Glutamylation of Taurine
However the availability of N-phthaloyl-L-glutamic anhydride (NPGLA) (Figure 8) in commercial quantities in high chemical and chiral purity has changed this scenario (11). The γ-glutamylation sequence becomes simpler and higher yielding when N-phthaloyl-L-glutamic anhydride is used.
As the γ-glutamylating agent. The amino acid, that needs to be γ-glutamylated, can be used directly without any protecting groups. A solvent system incorporating an aqueous polar solvent can be employed. The products are free from any possible racemization. Amines also can be employed on their conversion to γ-glutamyl derivatives. Finally phthaloyl group can be simply removed by standard methods such as use of hydrazine (see below Figure 9).
An improved synthesis of γ-L-glutamyl-taurine (Glutaurine) similarly involves the direct reaction of NPGLA (Figure 8) with taurine solubilized in acetonitrile with tetramethyl guanidine. Deprotection using hydrazine in the same pot afforded the dipeptide (Figure 10). In the synthesis of γ-glutamylmarasmine, NPGLA has been used effectively (12). An enantioselective synthesis of chloroquine and the establishment of configuration used NPGLA as a pivotal chiral agent (13).
Enzymatic methods of introduction of γ-glutamyl group have also been used (1). A bacterial γ-glutamyltranspepdidase enzyme was used. This enzymic technology still has to reach industrial levels since only low concentrations of substrates only could be used.
Deprotection of phthalimides (Removal of phthaloyl protecting group from primary amino function)
Variety of methods are available for the removal of the phthaloyl group to liberate the primary amine functionality. The classical and most widely employed method is through the use of hydrazine whereby the phthaloyl group is removed as phthalhydrazide or sometimes as N-aminophthalimide (14). The deprotection can be carried out in alcoholic solvents wherein the phthalhydrazide is easily removed as illustrated in Figure 9.
Sometimes co-occurring oxidation of hydrazine and the subsequent formation of diimide results in the reduction of double bonds present elsewhere in the substrate. To avoid such side-reactions, additionally a sacrificial olefin such as (Z)-2-buten-1-ol is employed that is more preferentially reduced to scavenge adventitious diimide (15). Also other hydrazines can be employed such as methylhydrazine where such diimide formation is averted (16). Alternatively phenylhydrazine can be employed in place of hydrazine. In stead of hydrazines, various primary amines including ammonia can be employed for the removal of phthaloyl group. Amines such as methyl amine, butyl amine, ethylene diamine or propylenediamine derivatives have been employed (14).
In addition to these basic amine reagents, acidic conditions have also been employed (14).
An interesting reductive removal of phthalimide protecting group using borohydride has been reported by Ganem et al. as illustrated in the Figure 11.
Ganem et al note that this reductive removal followed by acetic acid treatment was a one-flask operation and noracemization of the chiral centers was detected (17).
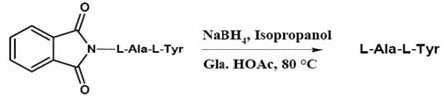
Figure 11. Reductive Removal of Phthaloyl Protecting Group
Conclusions
A readily available chiral γ-glutamyl transfer reagent, namely N-Phthaloyl-L-glutamic anhydride (NPGLA) onto amino acids and also onto primary amines, offers a simple solution for installing L-glutamyl functionality in a straightforward way. The amino acid need not bear any protecting groups and the reaction is free from racemization.
References and Notes
1. S. Wilk, H. Mizoguchi et al., J. Pharmacol. Exp. Ther. 206, p. 227 (1978)
2. H. Suzuki, Y. Kajimoto et al., J. Agri. Food Chem. 50, p. 313 (2002); H. Suzuki, K. Kato et al., J. Agri. Food Chem. 52, p. 577 (2004) and references therein
3. M. Friedman, P-A. Finot, J. Agri. Food Chem. 38, p. 2011 (1990)
4. J. Wu, H. Corke, J. Sci. Food Agri. 85, p. 2587 (2005)
5. A. Dunkel, J. Koster et al., J. Agri. Food Chem. 55, p. 6712, (2007)
6. N. Kalyanam, M. Majeed, Chem Oggi 25(5), p. 36 (2007)
7. Y. Dong, D. Lisk et al., Cancer Research 61, p. 2923 (2001)
8. J. Gulyas, F. Sebestyen et al., Organic Prep. Proced. Int. 19, p. 64 (1987)
9. H. Suzuki, K. Kato et al., J. Biotechnology 111, p. 291 (2004)
10. E. Block, M. Birringer et al., J. Agri. Food Chem. 49(1), p. 458 (2001); J. F. Carson, F. F. Wong, JCS Perkin I, p. 685 (1974); E. Khalifa, H. H. Bieri et al., Helv. Chim. Acta 56, p. 2911 (1973); M. Itoh, Chem. Pharm. Bull. 17, p. 1679 (1969)
11. Available from Sabinsa Corporation, www.sabinsa.com
12. L.A.G.M. Van den Broek, M. Breuer et al., J. Org. Chem. 52, p. 1511 (1987)
13. J. C. Craig, H. N. Bhargava et al., J. Org. Chem. 53, p. 1167 (1988)
14. For a brief review and leading references see, Greenes's Protecting Groups in Organic synthesis, P. G. Wuts, T. W. Greene, 4th Edition, John Wiley & Sons, Inc., Hoboken, NJ, pp 790-793 (2006)
15. B.E. Maryanoff, M.N. Greco et al., J. Am. Chem. Soc. 117, p. 1225 (1995)
16. A.L. Smith, C.K. Hwang et al., J. Am. Chem. Soc. 114, p. 3134 (1992)
17. J.O. Osby, M.G. Martin et al., Tetrahedron Lett. 25, p. 2093 (1984)










